Tapentadol, a central-acting analgesic, functions via a dual mechanism. This dual mechanism combines the mu-opioid receptor agonism (MOR), with the norepinephrine reuptake inhibition (NRI). Tapentadol represents the first of its kind in a new class of drugs, called the MOR-NRI agents [Freo et al., 2019].
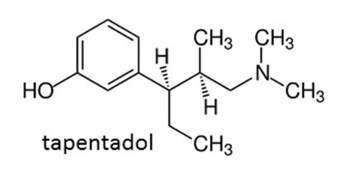
Figure 1. Chemical structure of Tapentadol. Tapentadol is considered a synthetic opioid. Image adapted from Franchi et al., 2019.