Summary
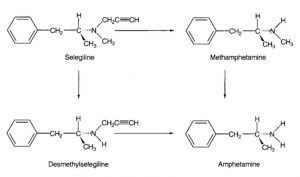
Selegiline (Figure 1) is a selective and irreversible mono-oxidase inhibitor, type B, (MAO-B) which is mainly used for the treatment of Parkinson’s disease (PD), as well as for depression [1; 2]. Selegiline was developed in the 1960s as a psychic energiser and is registered in more than 60 countries [3]. More recently, selegiline has been investigated as a possible therapy in ending tobacco smoking [4]. In this manner, selegiline was found to inhibit [mouse] nicotine metabolism [4]. In terms of treating depression, the selegiline transdermal system (skin patch system) was approved by the FDA (2006) for treatment of major depressive disorder (MDD) [5].
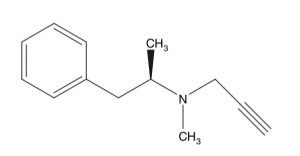
Figure 1


Reviews
There are no reviews yet.