Summary
Lunesta, also known as eszopiclone, was approved by the FDA in December, 2004, for the short-term treatment of insomnia [1].
Moreover, Lunesta is indicated for use in patients suffering from insomnia, above the age of 18 [1].
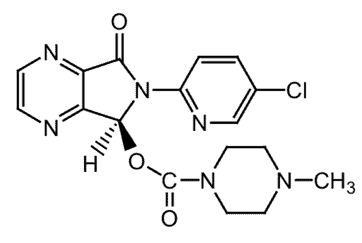
Figure 1. Lunesta, also known as eszopiclone, is a non-benzodiazepine hypnotic agent, with a molecular weight of 388.81.


Reviews
There are no reviews yet.