Summary
Tianeptine (Figure 1) is an antidepressant, currently marketed in 15 European Union (EU) countries, along with 66 countries across the world [1]. Tianeptine is considered a serotonergic nootropic, which equates to what is known as a ‘cognitive enhancer’ [2]. A cognitive enhancer is characterised by the following features: increasing circulation to the brain, offering precursors to the brain, improving neuron functioning, and preventing oxidative damage to cells of the brain [2]. In addition, the water soluble tianeptine sodium salt (TSS) is known as a facilitator of 5-HT (serotonin) and norepinephrine uptake [3]. Importantly, tianeptine sodium is an example of a nootropic incorporated into a new type of drug delivery system known as orally disintegrating films (ODFs) [4]. Clear advantages of an ODF delivery system compared to oral disintegrating tablets are rapid disintegration of drug, along with the avoidance of disadvantages associated with tablet drug delivery systems such as fragility, lower patient compliance and less surface area [5].
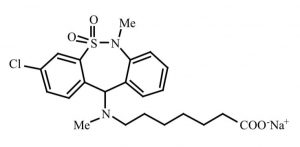
Tianeptine (Figure 1)


Reviews
There are no reviews yet.