Summary
Trazodone, [Fig.1] a triazolopyridine derivative belonging to the class of serotonin antagonist an reuptake inhibitors (SARI), is used in the treatment of depression [1] and is currently approved by the FDA to treat major depression [2]. Trazodone has been branded as Desyrel – trazadone hydrochloride (HCl), and its uses, besides its antidepressant activity, include treating medical and psychiatric disorders [3], along with the treatment of post-traumatic stress disorder (PTSD), insomnia, anxiety disorders, obsessive compulsive disorders (OCD), eating disorders, abuse of substances, impediments in cognitive functioning, sexual dysfunction, and particularly in rehabilitation following acute ischemic stroke [3; 4]. Specifically, trazodone HCl [Fig.1] is considered an anti-depressant agent. The way trazodone works is through its antagonism of the serotonin receptor (5-HT2A), alpha 1 and histamine H1 receptors, and this permits the drugs hypnotic effects (at low doses), whilst a high trazodone dose blocks serotonin transporter (SERT) and 5-HT2A allowing for its antidepressant activity [2]. In addition, due to trazodone’s strong antagonistic action at serotonergic type 2 (5-HT2) receptors it is known to quickly improve sleep [5; 1]. Further, trazodone’s pharmacological mode of action is such that a very low dose – 1mg trazodone – can block half of the brain’s 5-HT2A receptors [1].
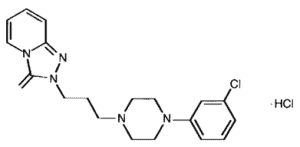
Figure 1. Structure and formula of trazodone. Trazodone (left) is a dual-acting antidepressant, able to inhibit the serotonin transporter (SERT) and serotonin type II (5-HT2) receptor [6]. Trazodone (C19H22CIN5O) molecular weight of 408.33 [7].


Reviews
There are no reviews yet.