Eszopiclone
$88.40 – $400.35Price range: $88.40 through $400.35
Shipping: 24h Dispatch | Fast ‘N Insured – AUS POST Ship | See details
Guarantee: Money Back Guarantee with Free Tracking | See details
Quality: Independent Product Analysis | View certificate
Payments: Buyer Protected via ![]() | See details
| See details
Summary
Lunesta, also known as eszopiclone, was approved by the FDA in December, 2004, for the short-term treatment of insomnia [1].
Moreover, Lunesta is indicated for use in patients suffering from insomnia, above the age of 18 [1].
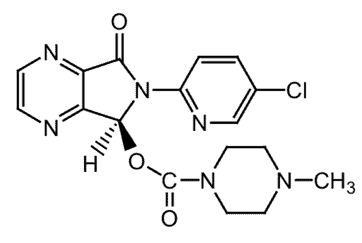
Figure 1. Lunesta, also known as eszopiclone, is a non-benzodiazepine hypnotic agent, with a molecular weight of 388.81.
Mechanism
The mechanism of action of Lunesta can be related to its effects on the GABA receptors [2].
Lunesta lasts around 6-8 hours in the body [1].
Lunesta is metabolised mainly in the liver by two mechanisms: oxidation and de-methylation [1].
Pharmacokinetics
Once taken, Lunesta (eszopiclone) is rapidly absorbed from the gastrointestinal (GI) tract within one hour [3].
Peak plasma concentrations of Lunesta are reached after around 1 hour of oral administration, and is binds weakly to plasma protein [4].
Lunesta & Anxiety
Lunesta (eszopiclone) has been previously investigated to determine its effects on treating insomnia comorbid with generalised anxiety disorder (GAD) [7].
Patients, aged 18-64, who fulfilled the criteria for anxiety, were administered eszopiclone and escitalopram (which is also an anti-anxiety drug).
Results of this work showed an improvement in total Hamilton Anxiety Scale scores, over 10 weeks [7].
Overall, researchers found improvements in not only sleep and daytime functioning, but also anxiety and mood in patients [7].
Dosage
Dosing with Lunesta should be done with your health professional.
As a guide, starting dose of Lunesta is between 1-2mg at bedtime [2].
Other sources recommend an initial dose of 1mg immediately before bedtime, and, for elderly individuals, Lunesta dose should not exceed 2mg [4].
Lunesta tablets are available in 1mg, 2mg and 3mg doses. Importantly, TOTAL dose of Lunesta should not exceed 3mg, once daily [4].
It is important to note that each Lunesta tablet contains 1mg, 2mg or 3mg eszopiclone (depending on the dose), with other inactive ingredients included in each tablet [4].
Drug Interactions
Lunesta may interact with other compounds, such as olanzapine which is a drug used to treat schizophrenia, depression and bipolar disorder [5].
Alcohol is another drug that can cause drug-drug interactions with Lunesta [3]. Other interactions may occur with central nervous system (CNS) depressants, and rifampicin, which is an antibiotic [6].
Side Effects
Side effects of Lunesta can include any one, or more of the following:
- Dizziness
- Dry mouth
- Difficulty maintaining coordination [8].
In most patients, Lunesta leaves a bitter taste in the mouth [3].
Serious side effects, such as hallucinations, memory loss, along with changes in mood, should be reported to your doctor immediately [8].
Lunesta Withdrawal
Withdrawal from Lunesta can occur as a result of mental and physical dependency on Lunesta [9]. Withdrawal symptoms can occur within 12 hours of discontinuing Lunesta, and so it is highly advisable to consult your doctor before quitting [9].
Most people experiencing Lunesta withdrawal will note difficulties in sleep after quitting the drug. Common withdrawal symptoms can include:
- Anxiety
- Irritability
- Tremors
- Mood swings
- Panic attacks [9].
Important Information
- Avoid taking more Lunesta than already prescribed.
- Do not take Lunesta unless you can maintain a 7-8 hour sleep cycle.
- Administer Lunesta immediately before going to bed.
- Contact your GP, or doctor, if insomnia has not improved within 7-10 days [10].
References
1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1325284/
2. https://www.aafp.org/afp/2005/0615/p2359.html
3. https://www.mdedge.com/psychiatry/article/66112/eszopiclone-targeting-chronic-insomnia
4. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021476s030lbl.pdf
5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC518985/
6. https://www.drugbank.ca/drugs/DB01045
7. https://www.ncbi.nlm.nih.gov/pubmed/18458207
8. https://www.webmd.com/drugs/2/drug-92350/lunesta-oral/details
9. https://www.addictioncenter.com/sleeping-pills/lunesta/withdrawal-detox/
10. https://www.lunesta.com/PostedApprovedLabelingText.pdf
Show reviews in all languages (1)
You must be logged in to post a review.


Reviews
There are no reviews yet.