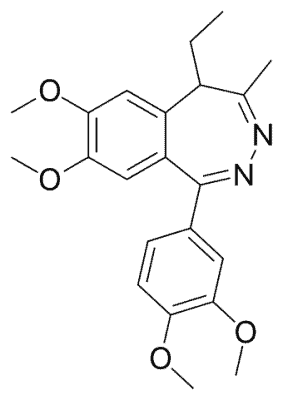
Tofisopam is a medication used to treat anxiety and alcohol withdrawal in several European countries. It belongs to the class of drugs known as benzodiazepines, but unlike other benzodiazepines, it does not have sedative, muscle relaxant, or memory-impairing effects. Tofisopam has been found to enhance the effectiveness of other anticonvulsant benzodiazepines such as diazepam and muscimol, but not other antiepileptic medications like sodium valproate, carbamazepine, phenobarbital, or phenytoin. The recommended dose of tofisopam is 50-300 mg per day taken in three divided doses, with peak levels reached in the blood two hours after ingestion. Although tofisopam is not thought to cause dependence as frequently as other benzodiazepines, it is still generally recommended to be used for a maximum of 12 weeks.


Reviews
There are no reviews yet.